
1. Physical Change: A change in which a substance undergoes a change in its physical properties is called physical change.
|
|
|
2. Chemical change: It is the change in which identity of the substance is changed
and a new substance is formed.
|
|
|
3. Signs of a chemical reaction: These factors denote that a chemical reaction has taken place- change of state of substance, change of colour of substance, evolution of heat, absorption of heat, evolution of gas and evolution of light.
|
|
|
4. Chemical reactions: The transformation of chemical substance into a new chemical substance by making and breaking of bonds between different atoms is known as Chemical Reaction.
 |
5. Chemical equation: The representation of chemical reaction by means of symbols of substances in the form of formulae is called chemical equation.
 |
6. Balanced chemical equation: A balanced chemical equation has number atoms of each element equal on both left and right sides of the reaction.
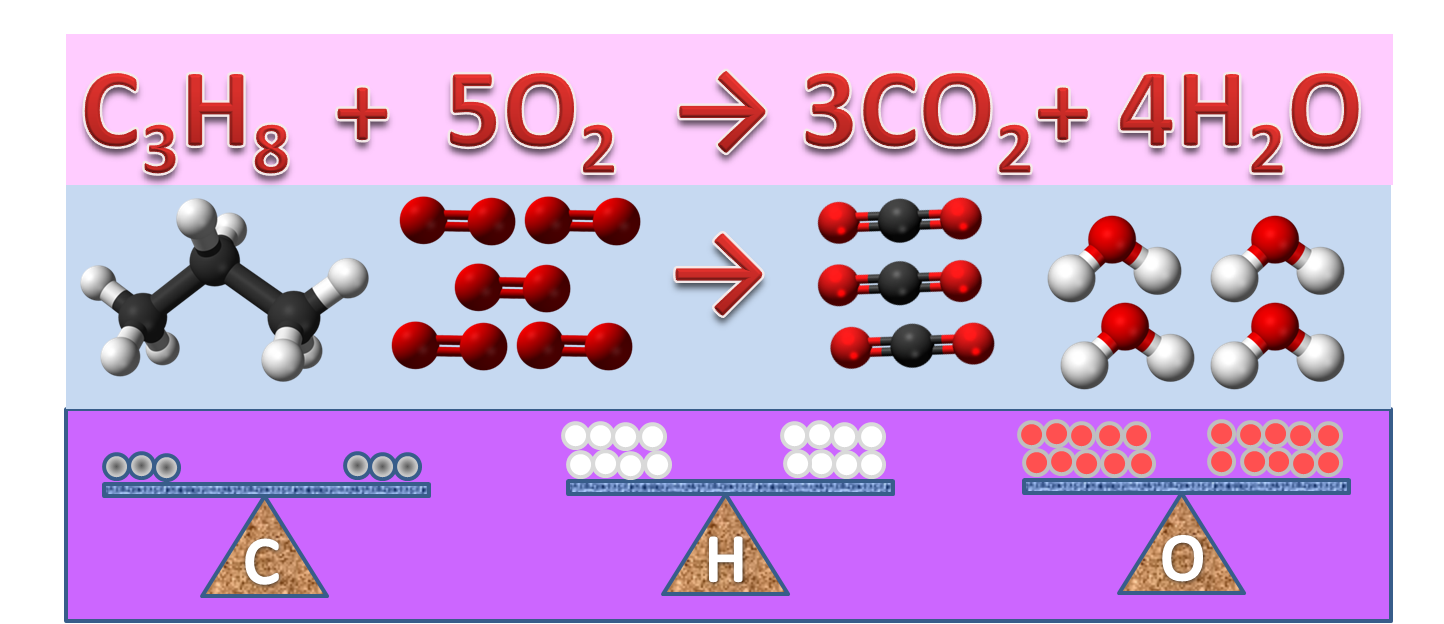 |
7. Combination reaction: When two elements or one
element and one compound or two compounds combines to give one single product.
 |
8. Decomposition reaction: Splitting of a compound into two or more simple products.
 |
9. Displacement reaction: It takes place when a more reactive metal displaces a
less reactive metal.
 |
10. Double displacement reaction: Reactions in which ions are exchanged between two reactants forming new compounds are called double displacement reactions.
 |
11. Precipitation: The insoluble compound called precipitate forms in this reaction.
 |
12. Exothermic: Reactions which produce energy are called exothermic reaction. Most of the decomposition reactions
are exothermic.
13. Endothermic- Reactions which absorb energy are called endothermic reaction. Most of the combination
reactions are endothermic.
 |
14. Oxidation: Gain of oxygen or removal of hydrogen or metallic element from a compound is known as oxidation.
15. Reduction: Addition of hydrogen or removal of oxygen from a compound is called reduction.
16. Redox reactions: A chemical reactions where oxidation and reduction both take place simultaneously are also known as redox reaction.
 |
17. Rusting: When Iron reacts with oxygen and moisture forms a red substance called rust.
 |
18. Rancidity: Oils and fats when oxidized on exposure to air show a change in taste and smell.
 |
19. Corrosion: Metals when attacked by oxygen, water, acids, and gases present in air changes their surface which is called corrosion.
 |









No comments:
Post a Comment